Manufacturer for Sofosbuvir - Pharmaceutical Grade 209216-23-9 Entecavir Monohydrate For Treatment of Chronic Hepatitis B – Yibai
Manufacturer for Sofosbuvir - Pharmaceutical Grade 209216-23-9 Entecavir Monohydrate For Treatment of Chronic Hepatitis B – Yibai Detail:
| Product name | Entecavir monohydrate |
| Synonyms | 2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate |
| CAS No. | 209216-23-9 |
| Appearance | White to off-white powder |
| Molecular Formula | C12H15N5O3.H2O |
| Molecular Weight | 295.30 |
| Usage | Pharmaceutical Grade or Research purpose |
| Packing | As per your request |
| Storage | Preserve in tight,light-resistant containers in a cool place |
|
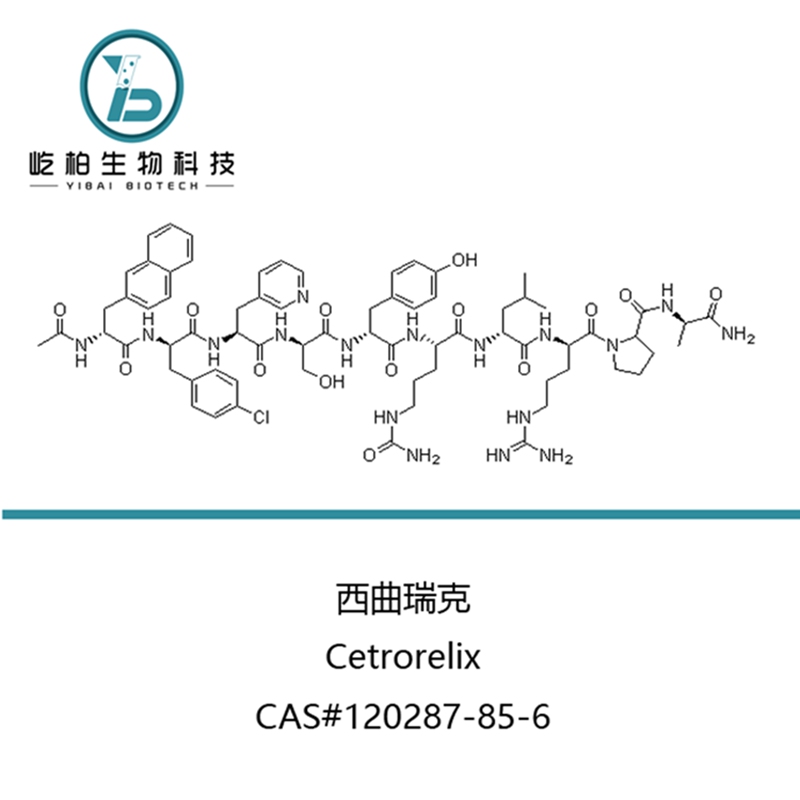
Entecavir monohydrate Cas: 209216-23-9 |
||
|
Items |
Standard |
Results |
| Appearance | White to off-white powder | Complies |
| Optical rotation | +24.0°—+28.0°(DMF:MeOH=1:1 c=1%) | +27.0° |
| Identification | A: By IR, To match with working standard | Complies |
| B: By HPLC,retain time should match with working standard | Complies | |
| Water | 5.8%—6.5% | 6.1% |
| Isomer | Single ≤0.1% | 0.02% |
| Total ≤0.5% | 0.02% | |
| Related Substances | Single impurity ≤0.1% | 0.05% |
| Total impurities ≤0.3% | 0.07% | |
| Residue on ignition | ≤0.1% | 0.04% |
| Heavy metals | £ 10ppm | Complies |
| Residual solvents | Should be to match with the standard | Complies |
| Assay | ≥99.8% | 99.9% |
| Conclusion:Complies to the in-house standards. | ||
Company Information
√ Management layer’s full experience in factory and skilled technicians followers; √ Quality is always our top consideration, Strict QC system; √ 11 years experienced exporting sales team; √ Independent R&D lab; √ Two signed long term GMP workshops; √ Rich resources of plenty idle factories for customized project; √ High Efficiency working team with consistent path.


Product detail pictures:


Related Product Guide:
Our merchandise are commonly recognized and reliable by customers and can meet constantly developing economic and social desires for Manufacturer for Sofosbuvir - Pharmaceutical Grade 209216-23-9 Entecavir Monohydrate For Treatment of Chronic Hepatitis B – Yibai , The product will supply to all over the world, such as: Japan, Brunei, Botswana, Our company regards "reasonable prices, efficient production time and good after-sales service" as our tenet. We hope to cooperate with more customers for mutual development and benefits. We welcome potential buyers to contact us.
The accounts manager made a detailed introduction about the product, so that we have a comprehensive understanding of the product, and ultimately we decided to cooperate.
Write your message here and send it to us