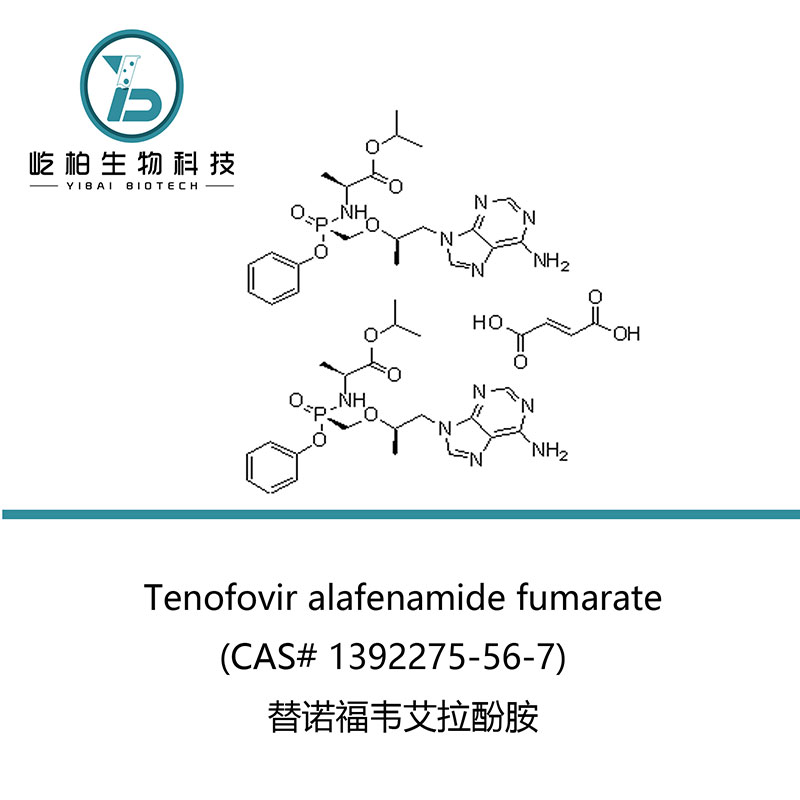
Chinese Professional Tenofovir Alafenamide Fumarate - USP 107910-75-8 Ganciclovir sodium for Antiviral – Yibai
Chinese Professional Tenofovir Alafenamide Fumarate - USP 107910-75-8 Ganciclovir sodium for Antiviral – Yibai Detail:
| Product name | Ganciclovir Sodium |
| Synonyms | 2-Amino-1,9-dihydro-9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]-6H-purin-6-one sodium salt |
| CAS No. | 107910-75-8 |
| Appearance | White or almost white crystalline powder |
| Molecular Formula | C9H13N5NaO4 |
| Molecular Weight | 278.22 |
| Usage | Pharmaceutical Grade or Research purpose |
| Packing | As per your request |
| Storage | Preserve in tight,light-resistant containers in a cool place |
|
Ganciclovir Sodium Cas: 107910-75-8 |
||
| Items | Standard | Results |
| Appearance | White or almost white crystalline powder | White crystalline powder |
|
Description |
White or almost almost white crystalline powder, odorless, hygroscopic |
Complies |
|
Solubility |
Soluble in water; very slightly soluble in methanol; practically insoluble in chloroform and acetone |
Complies |
| Identification | Ganciclovir Sodium is conformed by HPLCGanciclovir Sodium is conformed by UV spectrum
Sodium: A dense white precipitate is formed by reaction with potassium pyroantimoniate. The IR absorption spectrum of the preparation of the test specimen exhibits maxima only at the same wavelengths as that of a similar preparation of the standard. |
Complies Complies Complies
Complies |
|
Heavy metals |
Not more than 20ppm |
Complies |
|
Acetone |
Not more than 5000ppm |
Complies |
|
Residue on ignition |
Not more than 0.1% |
Complies |
| Related impurities | Not more than 0.5% for compound ANot more than 1.5% for total impurities |
0.32% 0.56% |
|
Water |
No more than 6.0% |
2.78% |
|
PH |
10.8~11.4 |
11.2 |
|
Assay |
Not less than 98.0% -102.0% (C9H13N5NaO4) |
99.4% |
| Conclusion: complies with USP40. | ||
Company Information
√ Management layer’s full experience in factory and skilled technicians followers; √ Quality is always our top consideration, Strict QC system; √ 11 years experienced exporting sales team; √ Independent R&D lab; √ Two signed long term GMP workshops; √ Rich resources of plenty idle factories for customized project; √ High Efficiency working team with consistent path.


Product detail pictures:


Related Product Guide:
Our firm sticks to the basic principle of "Quality is the life of your company, and status will be the soul of it" for Chinese Professional Tenofovir Alafenamide Fumarate - USP 107910-75-8 Ganciclovir sodium for Antiviral – Yibai , The product will supply to all over the world, such as: Ireland, Poland, Adelaide, As an experienced manufacturer we also accept customized order and we could make it the same as your picture or sample specification. The main goal of our company is to live a satisfactory memory to all the customers, and establish a long term business relationship with buyers and users all over the world.
The company can think what our think, the urgency of urgency to act in the interests of our position, can be said this is a responsible company, we had a happy cooperation!
Write your message here and send it to us